Introduction:
In general, the use of an immunomodulator (IMiD), proteasome inhibitors (PI) and dexamethasone (dex) for the treatment of MM is associated with better outcomes. The management of elderly patients with multiple myeloma (MM) is challenging due to difficulty in managing their co-morbidities and inability to tolerate treatment side effects. We evaluated therapies and outcomes of elderly patients with newly diagnosed MM.
Methods:
This is a retrospective study of patients with MM who were >75 years old treated at the Mayo Clinic, Rochester from January 2004 to January 2018. We included patients who were treated on clinical trials as well as off-trials. Patients were classified as receiving treatment with IMiD+PI+dex, alkylator+PI+steroid, IMiD+dex, PI+dex, alkylator+IMid+steroid, and other (alkylator with steroid or steroid only). Treatment response was documented as well as the progression-free (PFS), defined as the time from therapy initiation to disease relapse or death from any cause and overall survival (OS), defined as the time from start of treatment to death from any cause. A multivariate analysis for factors affecting OS was done including the following variables: being on a triplet combination (alkylator+PI+steroid, IMid+PI+dex, or alkylator +IMiD+steroid), revised international staging system (R-ISS)(stage 3 vs. 1-2), bone marrow plasma cell percentage (BMPC%)(>60% vs. ≤60%), and receiving treatment during or after 2010 vs. before 2010. Analysis was done for patients treated off-trials, as well as, including trial patients.
Results:
We identified 394 patients with MM who were >75 years old and 246 (62%) were male. For non-trial patients (n=350), IMiD+dex (32%) was the most commonly used regimen followed by alkylator with steroid or steroid only (20%), alkylator+PI+steroid (18%), and IMid+PI+dex (13%). The remaining patients were treated with PI+dex (12%) and alkylator +IMiD+steroid (5%). Forty-four patients (11%) were treated in clinical trials with alkylator+IMid+steroid (47%), IMiD+dex (25%), IMiD+PI+dex (14%), and alkylator+PI+steroid (14%). The median follow up was 45.9 months with an interquartile range of 28.2 to 75.6 months.
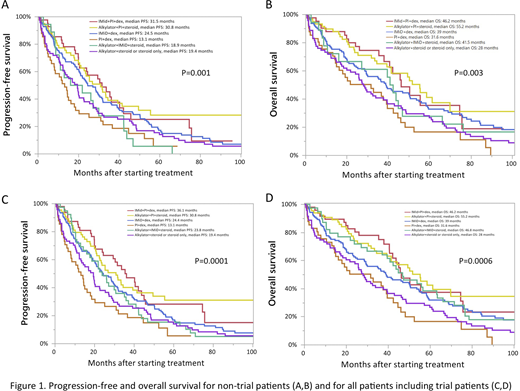
Overall, achieving very good partial response or complete response was more likely in patients who were treated with an IMid+PI+dex (58%) or alkylator+PI+steroid (47%), compared to in other therapies (5-30%)(P<0.0001). The PFS and OS for non-trial patients are displayed in Figure 1 (A,B) and for all, including trial patients in (C,D). Overall, the median OS was significantly longer in patients who were treated with a triplet in non-trial as well as all patients. In a multivariate for OS including non-trial patients, predictors for better OS included receiving a triplet (HR: 0.63, P=0.02) and not having an R-ISS stage 3 (HR: 0.39, P=0.001). This was also found when including trial patients (using a triplet, HR: 0.65, P=0.01 and not having an R-ISS stage 3, HR: 0.35, P=0.0002).
Conclusion:
In MM patients >75 years old, being able to receive triplet therapy is associated with better survival. This study provides better understanding of the natural history of MM outside of trials in the elderly age group.
Dispenzieri:Celgene: Research Funding; Alnylam: Research Funding; Pfizer: Research Funding; Intellia: Research Funding; Takeda: Research Funding; Janssen: Research Funding. Dingli:Bristol Myers Squibb: Research Funding; Alexion: Consultancy; Millenium: Consultancy; Rigel: Consultancy; Sanofi-Genzyme: Consultancy; Apellis: Consultancy; Janssen: Consultancy; Karyopharm Therapeutics: Research Funding. Kapoor:GlaxoSmithKline: Research Funding; Amgen: Research Funding; Takeda: Honoraria, Research Funding; Sanofi: Consultancy, Research Funding; Janssen: Research Funding; Cellectar: Consultancy; Celgene: Honoraria. Gertz:Spectrum: Other: personal fee, Research Funding; Janssen: Other: personal fee; Prothena: Other: personal fee; Alnylam: Other: personal fee; Ionis/Akcea: Other: personal fee; Springer Publishing: Patents & Royalties; Proclara: Other; DAVA oncology: Speakers Bureau; Johnson and Johnson: Speakers Bureau; Teva: Speakers Bureau; Sanofi: Other; Research to Practice: Other; Celgene: Other; Abbvie: Other; Aurora Bio: Other; Physicians Education Resource: Other: personal fee; Medscape: Other: personal fee, Speakers Bureau; Amgen: Other: personal fee; Appellis: Other: personal fee; Annexon: Other: personal fee. Kumar:Carsgen: Other, Research Funding; Tenebio: Other, Research Funding; BMS: Consultancy, Research Funding; Karyopharm: Consultancy; MedImmune: Research Funding; Sanofi: Research Funding; Novartis: Research Funding; Kite Pharma: Consultancy, Research Funding; Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Adaptive Biotechnologies: Consultancy; Merck: Consultancy, Research Funding; Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Dr. Reddy's Laboratories: Honoraria; Cellectar: Other; Genecentrix: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal